Antwort What are the 4 types of organic chemistry? Weitere Antworten – What are the 4 main elements of organic chemistry
Four elements, hydrogen, carbon, oxygen and nitrogen, are the major components of most organic compounds. Consequently, our understanding of organic chemistry must have, as a foundation, an appreciation of the electronic structure and properties of these elements.Specifically, the article focuses upon a core set of concepts that I call "the six pillars of organic chemistry": electronegativity, polar covalent bonding, inductive effects, steric effects, resonance, and aromaticity.There are four main types, or classes, of organic compounds found in all living things: carbohydrates, lipids, proteins, and nucleic acids.
What are the 5 main types of chemistry : Traditionally, the five main branches of chemistry are organic chemistry, inorganic chemistry, analytical chemistry, physical chemistry, and biochemistry.
What is 5 in organic chemistry
IUPAC numerical multiplier
| Number | Multiplier |
|---|---|
| 4 | tetra- |
| 5 | penta- |
| 6 | hexa- |
| 7 | hepta- |
What are the six pillars of organic chemistry : Specifically, the article focuses upon a core set of concepts that I call "the six pillars of organic chemistry": electronegativity, polar covalent bonding, inductive effects, steric effects, resonance, and aromaticity.
And thus, typically, we find (1) carbon, (2) hydrogen , (3) oxygen , (4) nitrogen , (5) phosphorus , and (6) sulfur .

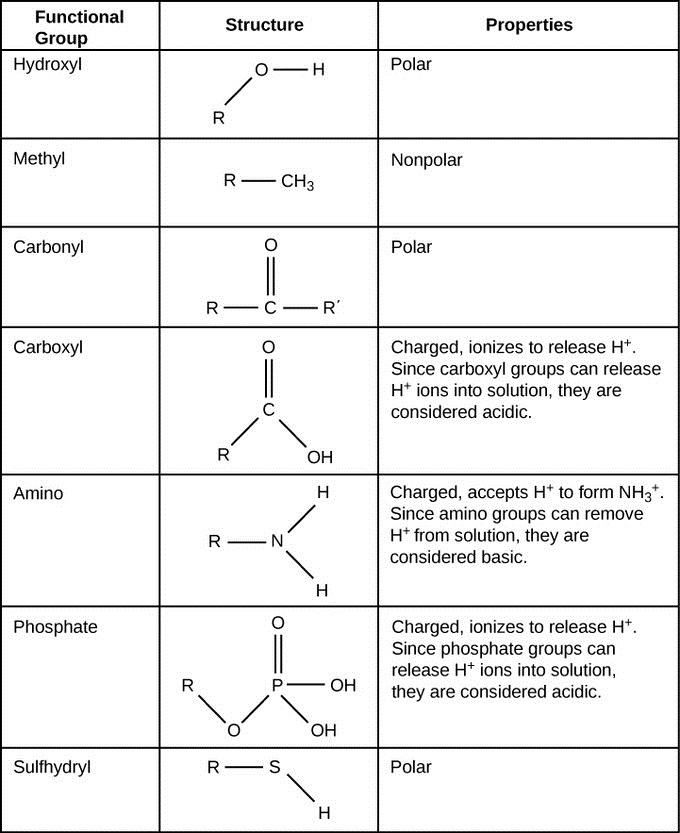
Key Takeaway. The four major classes of organic compounds found in biology are proteins, carbohydrates, lipids, and nucleic acids. Their structures and reactivity are determined by the functional groups present.
What are 5 examples of organic compounds
Examples of organic compounds are carbohydrates, fats (lipids), proteins, and nucleic acids, which are the basis for the molecules of life. Organic compounds also include petroleum and natural gas, which are the main components of fossil fuels.organic chemistry
Known for its complex concepts and demanding workload, organic chemistry is often considered one of the most difficult college classes.Fundamentals of Chemistry
- Front Matter.
- 1: Measurements.
- 2: Introduction to Chemistry and Science.
- 3: Matter.
- 4: The Periodic table.
- 5: Atoms and Molecules.
- 6: Molecules and Compounds.
- 7: Chemical Reactions and Equations.

| Number of Carbons | Name |
|---|---|
| 9 | nonane |
| 10 | decane |
| 11 | undecane |
| 12 | dodecane |
What is 9 called in organic chemistry : EXTENSION OF RULES A-1.1 AND A-2.5 CONCERNING NUMERICAL TERMS USED IN ORGANIC CHEMICAL NOMENCLATURE
| 1 | mono- or hen-* | deca- |
|---|---|---|
| 6 | hexa- | hexaconta- |
| 7 | hepta- | heptaconta- |
| 8 | octa- | octaconta- |
| 9 | nona- | nonaconta- |
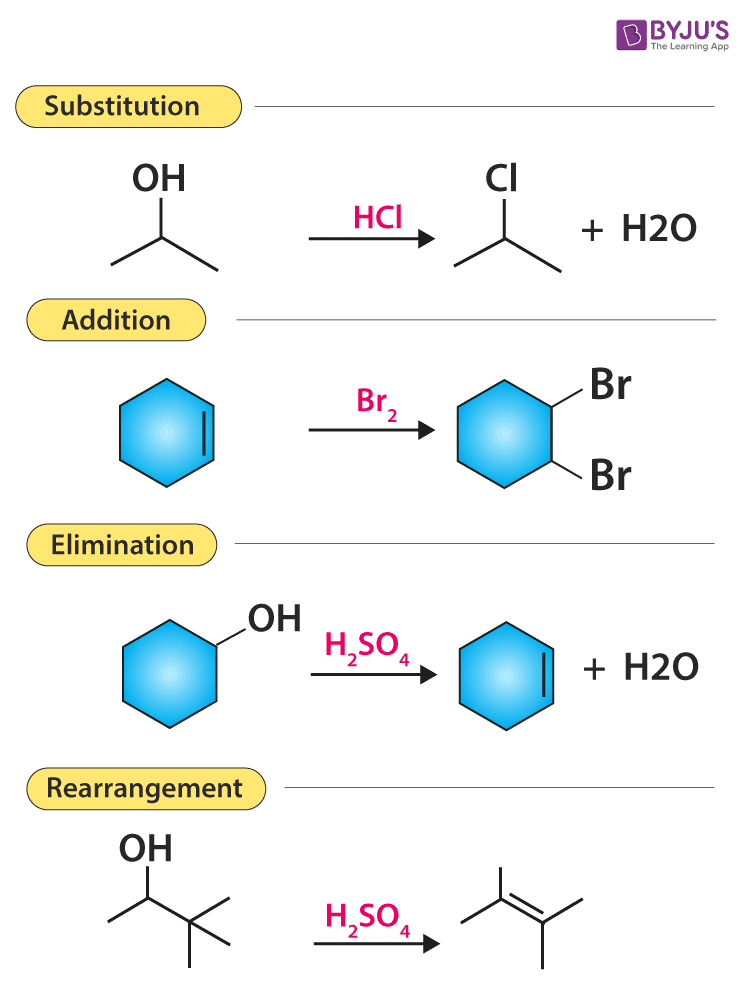
What are the seven golden rules of organic chemistry : An algorithm for filtering molecular formulas is derived from seven heuristic rules: (1) restrictions for the number of elements, (2) LEWIS and SENIOR chemical rules, (3) isotopic patterns, (4) hydrogen/carbon ratios, (5) element ratio of nitrogen, oxygen, phosphor, and sulphur versus carbon, (6) element ratio …
What are the 10 organic compounds
Organic compounds are a substance that contains covalently- bonded carbon and hydrogen and often with other elements. Organic compounds examples are benzoic Acid, aromatic compounds, benzoic aldehyde, propanoic acid, butanoic acid, malonic acid, amines, heterocyclic compounds, VOC, benzoic acid, and diethyl malonate.
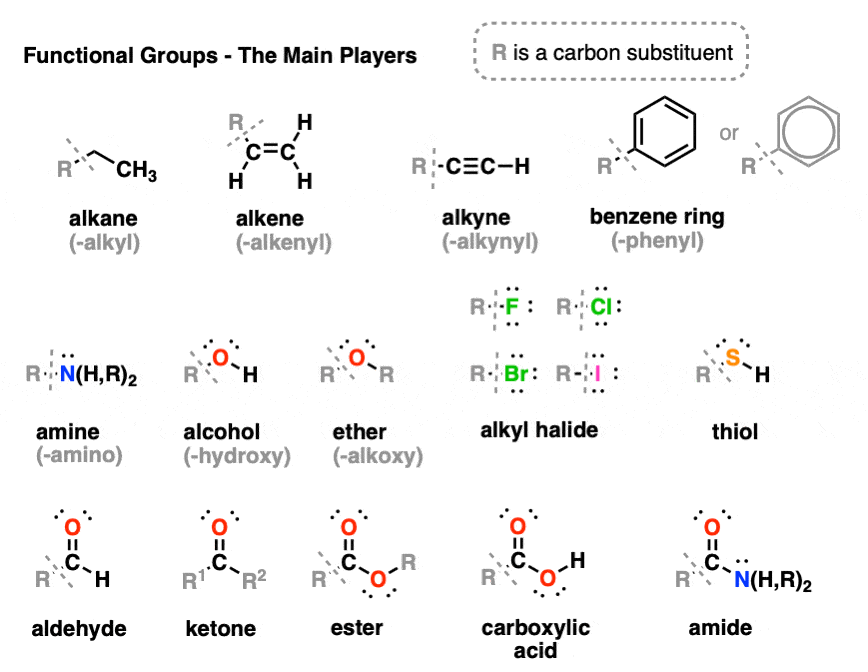
The CHNOPS elements are carbon, hydrogen, nitrogen, oxygen, phosphorus, and sulfur. These elements are the most abundant elements found in living things. Together, the six elements of life, form large, organic molecules known as macromolecules or biomolecules.These common products make use of organic chemistry:
- Shampoo.
- Gasoline.
- Perfume.
- Lotion.
- Drugs.
- Food and food additives.
- Plastics.
- Paper.
What is the rarest chemistry : Astatine
Astatine is a chemical element; it has symbol At and atomic number 85. It is the rarest naturally occurring element in the Earth's crust, occurring only as the decay product of various heavier elements. All of astatine's isotopes are short-lived; the most stable is astatine-210, with a half-life of 8.1 hours.



